All the players of the Special Cargo industry in one place

Up-to-date Capability
Platform
Support of GDP Compliancy

Data Explanation:
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum
Data Explanation:
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum
Today’s business environment is more dynamic than ever. So start to manage global distribution networks on our digital platform, securely and according to GAMP5 guidelines. Eliminate repetitive activities related to collecting supplier data and reduce the time it takes to create and approve Lane SOP's and Lane Assessments by at least 50 percent. That’s GDP compliance for the digital age.
Evaluate logistics supplier capabilities
Our Capabilities Platform provides direct access to the special handling capabilities of logistics providers, especially for air freight logistics. If the information you need is not yet available, simply send an invitation or data sharing request.
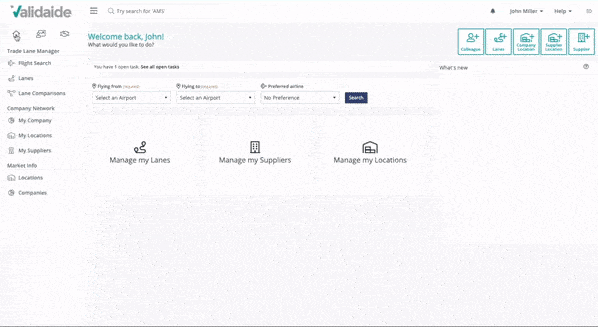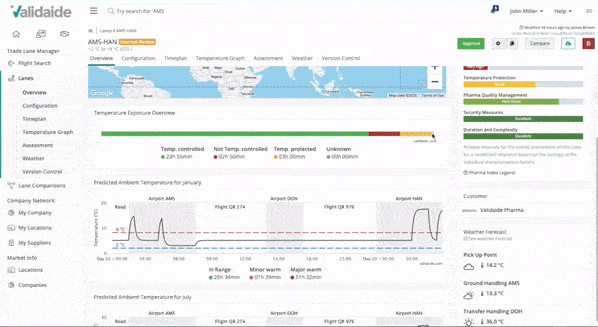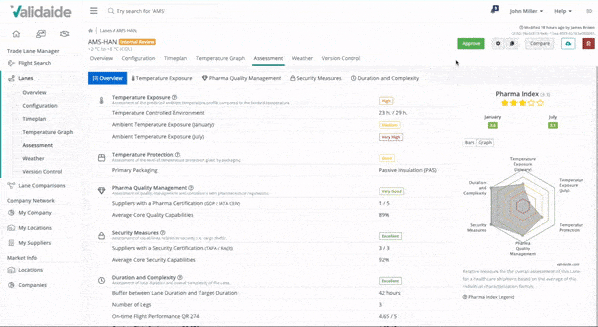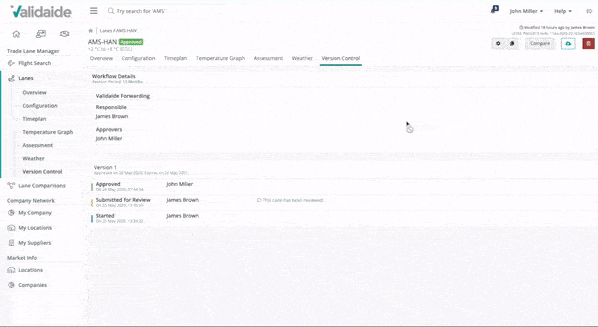
Create data-driven Lane Assessments
With just a few clicks, you can create a detailed transport time plan based on up-to-date supplier capabilities. Our application automatically combines these capabilities with climate data and provides a predicted temperature graph and a detailed Lane Assessment.
Manage and share digital Lane SOPs
Your Lane Assessments on Validaide can easily be combined into digital SOPs (Standard Operating Procedures). These SOPs automatically include contact details and provide flexible process descriptions for easy documentation. Forwarders can share the SOPs on our platform with their customers.
Approve with digital signatures
With Validaide, pharma manufacturers, freight forwarders and shippers can easily maintain and jointly approve SOPs and Lane Assessments with digital signatures. Revision periods and automatic notifications make it easy to maintain the information and a complete version history remains available.
Why Validaide?
Efficiency gains in regulatory compliance
Creating and maintaining Lane Assessment documents according to healthcare regulations often results in repetitive and redundant activities. With Validaide you can reduce these activities by 50% or more.

Faster Lane approvals
Setting up new transport routes can take considerable time as pharmaceutical shippers need to approve transport providers and processes first. With our data-driven approach and online approval workflow, you can accelerate this process.
Optimization of routing decisions
Easy access to up-to-date supplier capabilities helps you to make better-informed decisions about routing and thermal packaging. This increases the quality of
pharmaceutical logistics and helps you mitigate the risk related to temperature excursions and other deviations.
What our customers say
"The efficiency gain with Validaide is evident.
No more risk gambling, we now get a valuable and realistic output."
Astrid Thorissen
Global Healthcare Quality Manager

"Validaide allowed us to satisfy our customer and deliver +250 risk assessment within 4 days. It proves that Validaide is flexible and strives to find the right service for its customers."
Mark van Os de Man
Global Airfreight Product Manager Healthcare
“With Validaide, we can finally configure fact-based and data-driven lane assessment with a few clicks. This eliminates outdated Excel or subjective risk assessments.”
Patrick Preikschat
Director Life Science Europe

.png)


